The National Administration of Drugs, Food and Medical Technology (ANMAT), through the provisions 8616/2024 and 8664/2024 published this Thursday in the Official Gazette prohibited the sale, marketing and distribution of a counterfeit medical screw for implants and a series of blood pressure monitors with irregularities throughout the national territory. According to the agency, these products represent a risk for patients and health professionals, since they do not comply with the required quality standards.
ANMAT banned counterfeit medical screws
The first case that led to the intervention of ANMAT took place in Córdoba, where a counterfeit medical screw was discovered. This product is the “Kurosaka Screw ø6X30MM Titanium”, commonly used in surgical interventions for ligament fixation, such as in knee surgeries.
The counterfeit was detected during an inspection at the Onixel SRL company, where inspectors found a screw with a suspicious label. The screw is commonly used in surgical interventions for ligament fixation.
Provision 8616/2024 medical product implant ANMAT
The inspection at Onixel SRL took place on August 7, 2024. During the procedure, inspectors observed that one of the Kurosaka screws had a label that did not match the usual standards for legal medical products.
When requesting the relevant documentation to verify the origin of the product, the company was unable to provide the necessary papers to prove the purchase and origin of the screw. This lack of information increased suspicions about the authenticity of the product.
The ANMAT The agency stressed that the use of counterfeit medical products poses a serious risk to patients’ health. In this particular case, a surgical screw that does not meet quality standards can lead to complications during surgery, infections or even implant failure. Due to these risks, the agency has taken the decision to ban the marketing and use of the counterfeit Kurosaka screw throughout the country.
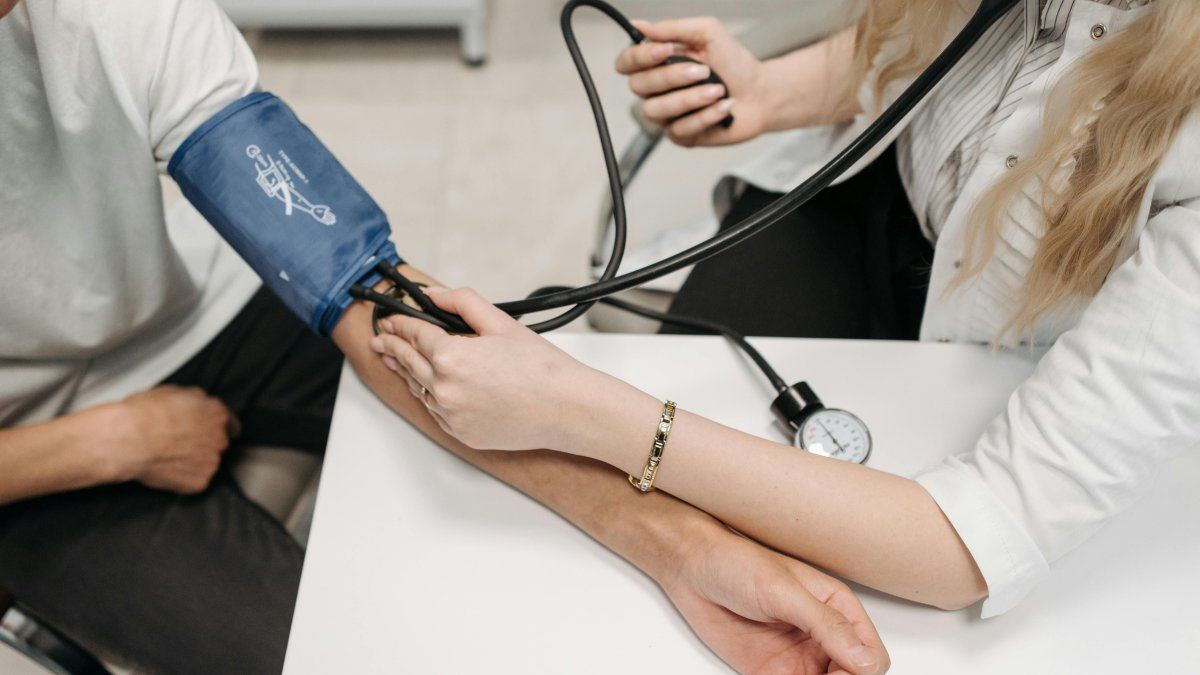
ANMAT banned the sale of a brand of blood pressure monitors
In a second case of relevance, the agency also prohibited the sale and use of a series of blood pressure monitors of the ALP-K2 brand due to irregularities in their documentation and origin. Blood pressure monitors are essential devices for measuring blood pressure, and their correct operation is essential to ensure accurate diagnoses.
The origin of the case dates back to July 2024, when ANMAT inspectors carried out a routine inspection at the company Productos para la Salud SRL, located in San Miguel de Tucumán. During this inspection, the inspectors found 12 ALP-K2 blood pressure monitors that did not comply with basic legal requirements.
ANMAT provision 8664/2024 prohibits the sale of blood pressure monitors
Among the irregularities detected, the products did not include a user manual in Spanish, something that is required under current regulations. The manufacturer or importer of the blood pressure monitors was also not clearly identified, which raised doubts about their legitimacy and origin.
The inspected company presented a purchase invoice indicating that the blood pressure monitors had been acquired through Trinidad Insumos SRL. However, when trying to verify this information, ANMAT discovered that Trinidad Insumos was no longer operating at the registered address, and it was not possible to confirm the origin of the products.
Source: Ambito
I am an author and journalist who has worked in the entertainment industry for over a decade. I currently work as a news editor at a major news website, and my focus is on covering the latest trends in entertainment. I also write occasional pieces for other outlets, and have authored two books about the entertainment industry.