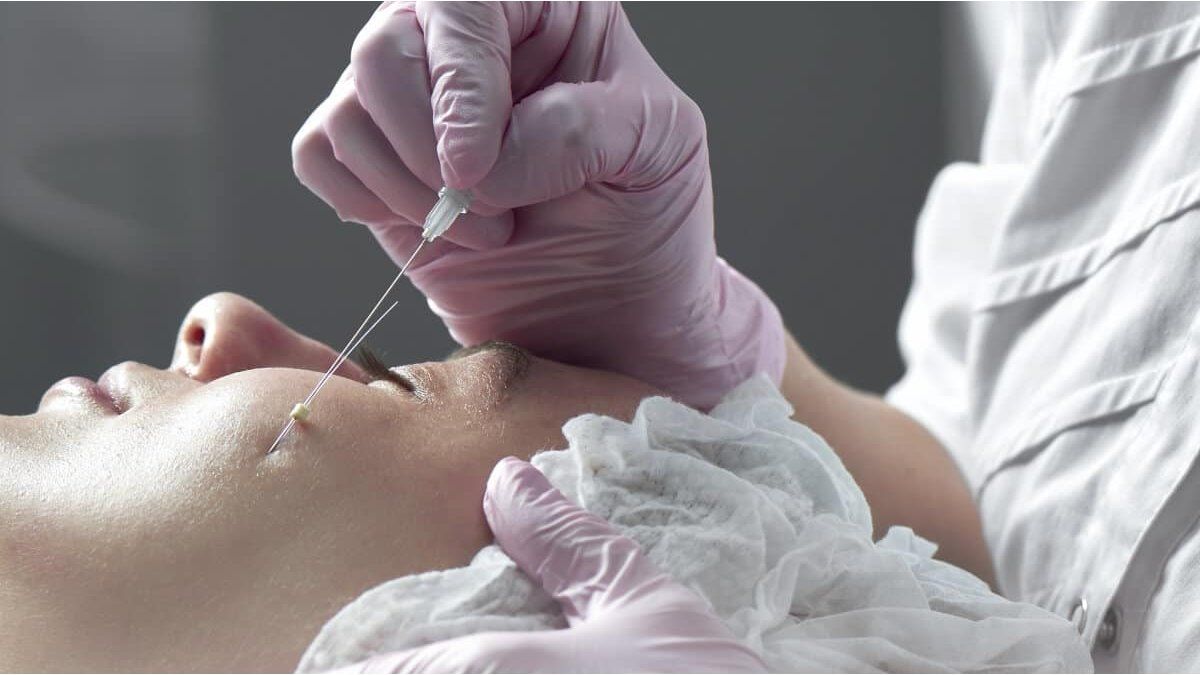
In detail, the administration carried out a search where products were detected without data from the responsible importer in Argentina or data from health authorizations, among other infractions. The measure was made official through the Publication of the provision 523/2025 in the Official Gazette.
Products prohibited by ANMAT
The measure was made official after the administration carried out the inspection of a premises in the Buenos Aires neighborhood of Belgrano, in an operation in charge of the Market Control Department of the Directorate of Evaluation and Management of Health Products Monitoring, Together with the City Police, Division Crimes against Health and Personal Safety. During the procedure Equipment and products were relieved, which showed several infractions.
Among the fouls detected, the ANMAT confirms that many products They did not have the Data from the responsible importer in Argentina or data from health authorizations, inscriptions in “Eastern language” and English and lack of lot identification and expiration date. In addition, several of the teams were falsified.
Being counterfeit medical elements and without traceability that could imply a risk to the health of the population, ANMAT decided to ban the following products: Lipo Laser & RF Slimming System Med-350manufacturer Beijing Kes Biology Technology Co. Ltd, Beijing, China; Sculptra 150 mg/5ml1 Vial, Sanofi Aventis Lot/Num: 5/2021; TKTX DEEP NUMBS NEW 10 GIngredient Lidocaine 5%. Prilocaine 5%. 1%Epinephrine; Minerva 20 PCs CNG Polydioxanone Suture – Made in Korea – Sterile EO; “CNG” Minerva Premium Pdo Suture – 20 pcs; CNG Polydioxanone SutureMade in Korea – Sterile Eo; Neodex 5 g Antibiotic & anti inflammatory combination; Li: Col Hard – Solid (Collagen) Prosthesis for noseDextran +PMMA / with Lidocaine, Manufacturer Chunghwa Medipower Co. Ltd; Gentamycin Sulfate Injection – Reyoung – Ampoules; Vanadium – Titanium crystal InjectionAesthetic Facial Restoration, Syringe Needle – Needle Tubing – Support, Made in Germany; Uverla – Presscog ThreadSterile Eo. 11. Uverla Cog Thread Sterile Eo.
Anmat prohibited a cinnamon of a famous Argentine brand for lead contamination
ANMAT also prohibited, days ago, the commercialization of a seasoning widely used in Argentine cuisine: The ground cinnamon of the “two anchors” brand. The measure was taken after organism authorities detected High levels of lead in certain lotswhich represents a risk to the health of the population.
The affected lots, identified as M270724, M290724 and M270824, correspond to 30 grams containers produced between July and August 2024. From the brand, it was reported that the withdrawal of the market was “volunteer“And that are replacing products to consumers.
The ground cinnamon lots prohibited by the ANMAT are the following:
-
M270724: Development date July 27, 2024.
-
M290724: Development date July 29, 2024.
-
M270824: Development date August 27, 2024.
Consumers who have any of these products in their possession should avoid their consumption and discard it immediately. This measure seeks Preserve the health and well -being of the population.
Source: Ambito
I am an author and journalist who has worked in the entertainment industry for over a decade. I currently work as a news editor at a major news website, and my focus is on covering the latest trends in entertainment. I also write occasional pieces for other outlets, and have authored two books about the entertainment industry.